Acidithiobacillus ferrooxidans: The Extremophile Revolutionizing Agriculture and Bioleaching
- Blessa Jana Samuel
- 4 hours ago
- 10 min read
Acidithiobacillus ferrooxidans stands as one of nature's most remarkable microorganisms, thriving in conditions where virtually no other life forms survive. This chemolithoautotrophic bacterium has earned significant attention in both agricultural and industrial biotechnology sectors for its extraordinary ability to oxidize ferrous iron and solubilize minerals in extremely acidic environments. Understanding the characteristics, metabolic processes, and genome insights of Acidithiobacillus ferrooxidans provides valuable knowledge for optimizing its applications in crop production, environmental remediation, and mining operations.
Characteristics and Morphology of Acidithiobacillus ferrooxidans
Acidithiobacillus ferrooxidans is a Gram-negative, non-spore-forming, chemolithoautotrophic rod-shaped bacterium with distinctive physical characteristics. The bacterium measures approximately 0.4 micrometers by 0.8 micrometers and typically appears as single cells or in pairs. Its taxonomic classification places it within the domain Bacteria, phylum Pseudomonadota, class Acidithiobacilla, order Acidithiobacillales, family Acidithiobacillaceae, and genus Acidithiobacillus.[1]
The name itself reflects the bacterium's key properties: "Acidithiobacillus" combines Latin and Greek roots—acidus (acidic), thios (sulfur), and bacillus (rod)—while "ferrooxidans" derives from ferrum (iron) and oxidare (to oxidize), collectively characterizing this organism's unique metabolic capabilities. Unlike most microorganisms that require organic compounds for survival, Thiobacillus ferrooxidans function depends entirely on inorganic chemicals, making it an extremophile perfectly adapted to the harsh conditions of acid mine drainage, iron-rich mineral deposits, and acidic agricultural soils.[1]
The bacterium's remarkable adaptability extends to its acid tolerance, maintaining internal pH homeostasis despite external conditions as acidic as pH 1.0 through specialized acid resistance mechanisms and proton pumps. This extreme tolerance allows Acidithiobacillus ferrooxidans to function in environments where most microorganisms cannot survive, positioning it as a critical player in both natural biogeochemical cycles and industrial processes.[2]
Genomic Profile and Molecular Architecture
Recent advances in genome sequencing have revolutionized our understanding of Acidithiobacillus ferrooxidans, revealing its remarkable metabolic complexity. The type strain ATCC 23270 contains a single circular chromosome comprising 2,982,397 base pairs with a G+C content of 58.77%, encoding approximately 3,217 protein-coding genes (CDSs). Some strains, such as YNTRS-40, possess even larger genomes reaching 3,257,037 base pairs with 3,349 CDS genes, including both chromosomal DNA and plasmid elements.[3][2]
The genome organization reflects the bacterium's metabolic sophistication. It contains two ribosomal operons and 78 transfer RNA (tRNA) genes, providing the molecular machinery for protein synthesis in its unique lifestyle. Notably, 64.3% of predicted genes have assigned putative functions, with the remaining genes representing novel or specialized metabolic functions. The presence of multiple tRNA synthetases, including both discriminating and non-discriminating glutamyl-tRNA synthetases, suggests complex regulatory mechanisms linking heme biosynthesis, nitrogen metabolism, and central carbon metabolism.[2]
The genomic analysis identified key functional clusters organized into transcriptional units, particularly the petI and rus operons critical for iron oxidation processes. These genetic arrangements enable the bacterium to efficiently coordinate electron transport and energy generation from inorganic substrates—a capability unmatched among most free-living organisms.[2]
Metabolic Processes: Energy Generation and Biochemical Innovation
The metabolic versatility of Acidithiobacillus ferrooxidans fundamentally distinguishes it from heterotrophic organisms. As a chemolithoautotroph, the bacterium generates energy through oxidative phosphorylation using inorganic compounds as electron donors while fixing atmospheric CO₂ as its sole carbon source via the Calvin cycle. This unique metabolic strategy enables survival in nutrient-poor, extreme environments where organic substrates are unavailable or inhibitory.[4]
Iron Oxidation Pathway
Thiobacillus ferrooxidans function centers primarily on iron oxidation, employing a sophisticated electron transport system that accelerates ferrous iron oxidation rates approximately 500,000 times faster than abiotic processes. The oxidation pathway features rusticyanin, a unique blue copper protein (encoded in the rus operon) that facilitates the oxidation of Fe²⁺ to Fe³⁺. This process generates adenosine triphosphate (ATP) through oxidative phosphorylation while producing ferric iron that solubilizes various mineral compounds.[5][6][7][8][2]
The electron transport chain during iron oxidation operates through dual pathways: a "downhill electron pathway" directing electrons through c-type cytochrome Cyc1 to aa₃-type cytochrome oxidase, and an "uphill electron pathway" regenerating the universal electron donor NADH through reverse electron flow via the bc₁ complex and ubiquinone pool. This dual-pathway system maximizes energy capture from the limited electrochemical potential of ferrous iron oxidation.[2]
Sulfur Oxidation Networks
Beyond iron oxidation, Acidithiobacillus ferrooxidans utilizes multiple sophisticated sulfur oxidation pathways. The bacterium employs the sulfur dioxygenase (SDO) system to initiate elemental sulfur oxidation, combined with complex thiosulfate oxidation mechanisms involving tetrathionate hydrolase and sulfite oxidase enzymes. These interconnected pathways provide metabolic flexibility, allowing the organism to switch between energy substrates depending on environmental availability.[9][2]
Under aerobic conditions, the bacterium catalyzes complete oxidation reactions. For example, ferrous sulfate (FeSO₄) oxidation produces ferric sulfate and sulfuric acid, generating the highly acidic environments characteristic of acid mine drainage. Under anaerobic or micro-aerophilic conditions, the bacterium demonstrates remarkable metabolic flexibility, utilizing ferric iron (Fe³⁺) or elemental sulfur as alternative electron acceptors.[2]
Central Carbon Metabolism and CO₂ Fixation
The Calvin cycle serves as the primary carbon fixation pathway in Acidithiobacillus ferrooxidans, with the genome encoding complete pathways for CO₂ fixation via ribulose-1,5-bisphosphate carboxylase. Fixed carbon enters the Embden-Meyerhof-Parnass pathway, channeling products toward either glycogen biosynthesis for energy storage or anabolic reactions for cellular building blocks.[4]
The bacterium's carbon metabolism includes genes for glucose-6-phosphate metabolism, glycogen storage and mobilization through glucan phosphorylases, and pyruvate regeneration. This metabolic architecture enables rapid response to fluctuating energy availability while maintaining sufficient biosynthetic capacity for growth and maintenance in minimal nutrient environments.[2]
Specialized Metabolic Capabilities
Recent genomic analysis revealed that Acidithiobacillus ferrooxidans possesses genes encoding respiratory hydrogenase complexes and hydrogen-evolving complexes, indicating capacity for hydrogen metabolism. Additionally, the genome contains predictive evidence for anaerobic sulfur reduction, hydrogen metabolism, and potentially nitrogen fixation—capabilities that expand its ecological niche and industrial applications beyond traditional iron and sulfur oxidation.[9][2]
Biofilm Formation and Cellular Processes
The bacterium forms protective biofilms that enhance survival in harsh conditions and improve efficiency in bioleaching applications. These biofilms involve extracellular polymeric substances (EPS) that facilitate cooperative interactions with other beneficial microorganisms and enable bacterial attachment to mineral surfaces. The biofilm matrix creates localized acidic microenvironments that accelerate mineral dissolution and nutrient release—a critical mechanism in both agricultural and industrial applications.[10][2]
The bacterium maintains complex stress responses, DNA repair mechanisms, and metal homeostatic systems encoded within its genome, allowing survival in environments with dissolved metal concentrations as high as 10⁻¹ M (compared to 10⁻¹⁶ M in typical neutrophilic environments). These heavy metal resistance mechanisms position uses of Acidithiobacillus ferrooxidans in bioremediation and waste processing applications where high contaminant concentrations would inhibit most other microorganisms.[2]
Agricultural Applications: Solving Iron Deficiency Chlorosis
One of the most significant applications of Acidithiobacillus ferrooxidans in agriculture addresses iron deficiency chlorosis (IDC), a widespread problem affecting crop productivity in approximately 30% of the world's cultivated soils, particularly in calcareous regions. In these high-pH environments, iron precipitates as insoluble ferric hydroxide, becoming unavailable for plant uptake despite adequate total iron content in the soil.[11]
Iron Solubilization and Plant Nutrient Availability
Acidithiobacillus ferrooxidans acts as a natural biofertilizer by continuously converting unavailable iron forms into plant-accessible nutrients through its iron-oxidizing metabolism. Field studies demonstrate remarkable improvements in crop growth parameters when iron-solubilizing bacterial treatments are applied: shoot length increases by 58%, root length by 54%, and iron concentration in plant tissues by 79%. These improvements occur because the ferric iron produced during bacterial oxidation of ferrous iron solubilizes mineral compounds in soil, enhancing bioavailability of iron and other micronutrients.[12]
The bacterium's activity particularly benefits crops grown in iron-deficient or alkaline soils, including cereals, millets, pulses, oilseeds, vegetables, fruits, and ornamental crops. Unlike chemical iron fertilizers that provide temporary nutrient boosts, Acidithiobacillus ferrooxidans establishes long-term soil health improvements by continuously converting unavailable iron forms, reducing dependence on synthetic inputs.
Enhanced Root Development and Stress Tolerance
Through improved iron availability and soil structure enhancement, uses of Acidithiobacillus ferrooxidans extend to promoting extensive root system development. Stronger root systems improve water and nutrient uptake capacity, leading to more resilient crops. Plants colonized by this bacterium demonstrate improved tolerance to abiotic stresses including drought, salinity, and nutrient deficiency conditions—benefits particularly valuable in challenging growing environments facing climate variability.
Industrial and Environmental Applications
Beyond agriculture, Acidithiobacillus ferrooxidans demonstrates transformative potential in multiple industrial sectors, fundamentally advancing biomining, environmental remediation, and waste processing technologies.
Bioleaching of Rare Earth Elements
Recent breakthroughs demonstrate uses of Acidithiobacillus ferrooxidans in recovering rare earth elements (REEs) from multiple mineral sources with superior efficiency compared to conventional chemical methods. In ion-adsorption type rare earth ore bioleaching, the bacterium achieved extraction rates surpassing current industrial standards: lanthanum (99.5%), neodymium (95.8%), and yttrium (93.5%)—exceeding conventional ammonium sulfate leaching by 23.1%, 23.4%, and 13.8%, respectively.[13]
The bioleaching mechanism operates through dual processes: direct contact between bacteria and mineral surfaces, and Fe²⁺ oxidation generating acid and ferric iron that facilitate proton exchange reactions. The bacterium's extracellular polymeric substances form complexes with rare earth ions, enhancing element release from REE-bearing minerals. Research on phosphate rock bioleaching revealed that Acidithiobacillus ferrooxidans elevated REE leaching rates by approximately 50% compared to abiotic leaching processes, achieving total leaching rates of 28.46% for mixed REEs.[14][15][13]
Heavy Metal Remediation
Thiobacillus ferrooxidans function extends to environmental remediation applications, particularly for treating contaminated sewage sludge and industrial waste streams. In controlled bioleaching experiments on dewatered sewage sludge, the bacterium achieved metal extraction rates of 42% zinc (1,300-1,648 mg/kg), 39% copper (613-774 mg/kg), and 10% chromium (37-44 mg/kg) over a 40-day period. This selective metal solubilization enables removal of hazardous elements before agricultural land application of sludge materials.[16]
Combined applications with biochar demonstrated particularly promising results: Acidithiobacillus ferrooxidans combined with biochar reduced soil heavy metal content by 28.42% and crop contamination by 60.82%—addressing critical environmental concerns in mining-affected and contaminated agricultural regions.[12]
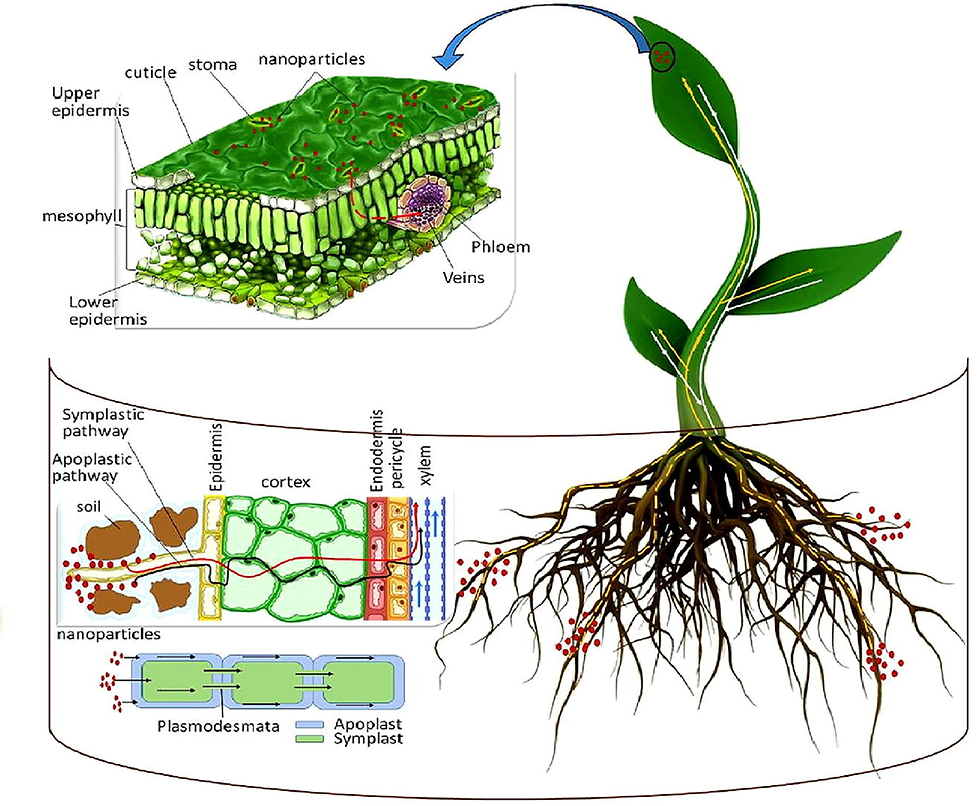
Nanoparticle Synthesis and Advanced Materials
The bacterium's unique ability to synthesize magnetite (Fe₃O₄) nanoparticles under mild conditions offers biotechnological advantages for biomedical and materials science applications. These biogenic nanoparticles possess superior properties compared to chemically synthesized alternatives, with potential applications in drug delivery, biosensing, and environmental remediation technologies.[12]
Genetic Engineering and Process Optimization
Advances in genetic modification have enhanced Acidithiobacillus ferrooxidans capabilities for specialized applications. Engineered strains demonstrate up to 13-fold improvements in lanthanide recovery efficiency compared to wild-type organisms. Additionally, process optimization using metallic iron instead of iron sulfate in growth media has simplified and improved commercial production processes, reducing costs and environmental impacts associated with industrial cultivation.[12]
Practical Application and Management in Agricultural Systems
Compatibility and Storage
Acidithiobacillus ferrooxidans maintains compatibility with bio-pesticides, bio-fertilizers, and plant growth hormones but should not be used simultaneously with chemical fungicides or pesticides that harm microbial viability. The product maintains stability for up to one year from manufacturing when stored in cool, dry conditions away from direct sunlight. Under favorable field conditions, the bacterium remains active for extended periods throughout the growing season.
Future Perspectives and Conclusion
The convergence of genomic insights, improved cultivation techniques, and expanding application domains positions Acidithiobacillus ferrooxidans at the forefront of sustainable agriculture and industrial biotechnology. As research continues to uncover the complexity of its metabolic capabilities and the potential of genetically optimized strains, this extremophile bacterium promises solutions to pressing global challenges: agricultural sustainability in iron-deficient soils, environmental remediation of metal-contaminated ecosystems, and sustainable recovery of critical minerals essential for modern technologies.
Acidithiobacillus ferrooxidans exemplifies how understanding microbial extremophiles at molecular and genomic levels translates into practical innovations. From traditional agricultural applications addressing nutrient deficiencies to cutting-edge applications in rare earth element recovery and environmental remediation, this bacterium demonstrates the untapped potential within Earth's microbial communities for addressing contemporary challenges in food security, environmental protection, and resource sustainability.
References
IndoGulfBioAg. (2025). Acidithiobacillus ferrooxidans - Microbial Species. Retrieved from https://www.indogulfbioag.com/microbial-species/acidithiobacillus-ferrooxidans[12]
Valdés, J., Pedroso, I., Quatrini, R., Dodson, R. J., Tettelin, H., Blake, R., ... & Holmes, D. S. (2008). Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genomics, 9(1), 597. https://pmc.ncbi.nlm.nih.gov/articles/PMC2621215/[2]
Functional analysis of gapped microbial genomes: Amino acid metabolism in Acidithiobacillus ferrooxidans. (2000). Proceedings of the National Academy of Sciences, 97(7), 3509-3514. https://www.pnas.org/doi/10.1073/pnas.97.7.3509[4]
Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans. (2019). PubMed Central. https://pubmed.ncbi.nlm.nih.gov/30919119/[17]
Valdés, J., Pedroso, I., Quatrini, R., Dodson, R. J., Tettelin, H., Blake, R., ... & Holmes, D. S. (2008). Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genomics. https://pubmed.ncbi.nlm.nih.gov/19077236/[18]
Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans. (2024, November 22). Microorganisms, 12(11). https://pmc.ncbi.nlm.nih.gov/articles/PMC11678928/[19]
Extending the models for iron and sulfur oxidation in the extremely acidophilic archaeon Metallosphaera sedula. (2009). PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC2754497/[20]
Zhang, Y., et al. (2019). Complete Genome Sequence of Acidithiobacillus ferrooxidans Strain YNTRS-40. Journal of Genomic Medicine, 5(1). https://pmc.ncbi.nlm.nih.gov/articles/PMC7023503/[3]
Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. UC eScholarship. https://escholarship.org/uc/item/4bq1h1rn[21]
Iron oxidation by Thiobacillus ferrooxidans. Oregon State University Digital Repository. https://ir.library.oregonstate.edu/downloads/6t053k34d[8]
Characterization and genomic analysis of two novel acidophilic iron-oxidizing Acidithiobacillus strains. (2022). Frontiers in Microbiology, 13, 960324. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.960324/full[22]
Acidithiobacillus ferrooxidans. (2010). Wikipedia. https://en.wikipedia.org/wiki/Acidithiobacillus_ferrooxidans[1]
Bioleaching and mechanism of ion-adsorption type rare earth ore by Acidithiobacillus ferrooxidans. (2025). Hydrometallurgy, 269. https://www.sciencedirect.com/science/article/abs/pii/S0304386X2500060X[13]
Olson, G. J., Brierley, J. A., & Brierley, C. L. (2003). Bioleaching of heavy metals from dewatered sludge by Acidithiobacillus ferrooxidans. Journal of Chemical Technology & Biotechnology, 78(4), 411-418. https://onlinelibrary.wiley.com/doi/10.1002/jctb.1330[16]
Deconstructing the genetic architecture of iron deficiency chlorosis in plants. (2020). Plant Molecular Biology, 102, 35-52. https://pmc.ncbi.nlm.nih.gov/articles/PMC6988307/[11]
Bioleaching of rare-earth elements from phosphate rock using Acidithiobacillus ferrooxidans. (2022). Letters in Applied Microbiology, 75(5), 1111-1125. https://academic.oup.com/lambio/article/75/5/1111/6989459[14]
Effects of dissolved low molecular weight organic acids on bioleaching of rare earth elements. (2008). Hydrometallurgy, 91(3-4), 139-150. https://www.sciencedirect.com/science/article/abs/pii/S0304389408006870[23]
Iron nutrition management in calcisol soils as a tool to mitigate iron deficiency chlorosis. (2020). Journal of Applied Biology & Biotechnology, 8(6). https://jabonline.in/abstract.php?article_id=1053&sts=2[24]
Bioleaching of rare-earth elements from phosphate rock using Acidithiobacillus ferrooxidans. (2022). PubMed. https://pubmed.ncbi.nlm.nih.gov/35611559/[15]
Heavy metal resistance strategies of acidophilic bacteria in acid mine drainage. (2014). Applied Microbiology & Biotechnology, 98(21), 8845-8857. https://pubmed.ncbi.nlm.nih.gov/24510139/[25]
Hazra, T. K., Mukherjea, M., & Mukherjea, R. N. (1992). Role of rusticyanin in the electron transport process in Thiobacillus ferrooxidans. Indian Journal of Biochemistry & Biophysics, 29(1), 77-81. https://pubmed.ncbi.nlm.nih.gov/1592418/[26]
Rusticyanin electron transport chain function. (2012). Wikipedia. https://en.wikipedia.org/wiki/Rusticyanin[5]
Vargas-Straube, M. J., Beard, S., Norambuena, R., Paradela, A., Vera, M., & Jerez, C. A. (2020). High copper concentration reduces biofilm formation in Acidithiobacillus ferrooxidans by decreasing production of extracellular polymeric substances and its adherence to elemental sulfur. Applied & Environmental Microbiology, 86(15), e00942-20. https://www.sciencedirect.com/science/article/pii/S1874391920302426[27]
Cox, J. C., & Boxer, D. H. (1978). The purification and some properties of rusticyanin, a blue copper protein involved in iron(II) oxidation from Thiobacillus ferro-oxidans. Biochemical Journal, 174(3), 497-502. https://pmc.ncbi.nlm.nih.gov/articles/PMC1185940/[6]
Extracellular polymeric substances mediate bioleaching of metal sulfide minerals. (2006). Applied Microbiology & Biotechnology, 72(2), 200-207. https://pubmed.ncbi.nlm.nih.gov/16431087/[28]
Fariq, A., Yasmin, A., Nasim, F. U. H., Khan, S., Zaman, W., & Lindström, E. B. (2019). Whole genome sequence analysis reveals high genetic plasticity and novel features of Acidithiobacillus ferrooxidans IO-2C. Scientific Reports, 9, 13128. https://www.nature.com/articles/s41598-019-49213-x[9]
Keywords: Acidithiobacillus ferrooxidans, Thiobacillus ferrooxidans function, uses of Acidithiobacillus ferrooxidans, bioleaching, iron-solubilizing bacteria, extremophile microorganisms, biofertilizer, rare earth element recovery, heavy metal remediation, agricultural biotechnology.
⁂
https://en.wikipedia.org/wiki/Acidithiobacillus_ferrooxidans
https://www.indogulfbioag.com/microbial-species/acidithiobacillus-ferrooxidans
https://www.sciencedirect.com/science/article/abs/pii/S0304386X2500060X
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.960324/full
https://www.sciencedirect.com/science/article/abs/pii/S0304389408006870
https://www.sciencedirect.com/science/article/pii/S1874391920302426
https://www.sciencedirect.com/science/article/abs/pii/S1874391920302426

Comments