How Trichoderma spp. Trigger Plant Systemic Resistance to Fusarium: Molecular Mechanisms and Signaling Pathways
- Stanislav M.

- Aug 7, 2025
- 7 min read
Updated: Sep 8, 2025
Trichoderma species represent one of agriculture's most significant biological innovations, functioning as versatile soil fungi that simultaneously serve as biocontrol agents, biofertilizers, and soil health enhancers across virtually all major crop systems worldwide.
These naturally occurring microorganisms have achieved remarkable agricultural success through their multifunctional approach to crop improvement, delivering tangible benefits by suppressing major soil-borne pathogens (achieving 60-80% disease reduction against Fusarium, Rhizoctonia, Pythium, and Phytophthora), enhancing nutrient availability through phosphorus solubilization and hormone production, and improving soil structure and microbial diversity.
Trichoderma species employ multiple sophisticated mechanisms, including mycoparasitism (directly attacking pathogenic fungi through enzymatic degradation), antibiosis (producing antimicrobial compounds), competition for nutrients and space, and induced systemic resistance (activating plant defence pathways via jasmonic acid, ethylene, and salicylic acid signalling).
Beyond pathogen control, they function as phosphate-solubilising microorganisms, converting insoluble soil phosphorus into bioavailable forms while producing plant growth hormones like auxins and gibberellins that stimulate root development and enhance nutrient uptake efficiency.
Their ability to enable yield increases of 20-60% across diverse crops while reducing chemical pesticide reliance by up to 50% makes Trichoderma a scientifically-backed biological solution that bridges environmental stewardship with agricultural productivity, representing a natural revolution that works with biological processes to enhance crop resilience, soil health, and sustainable farming systems.
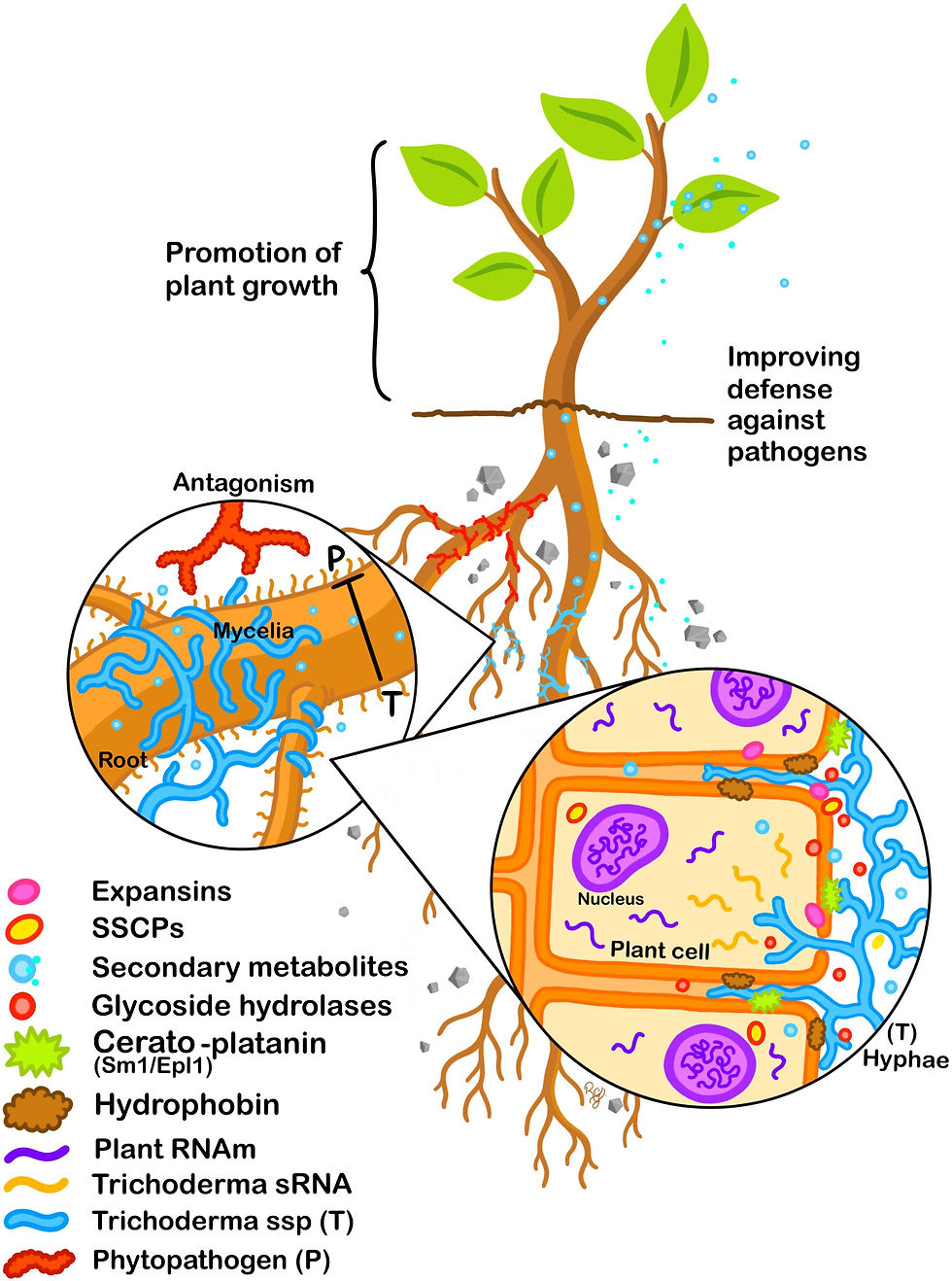
The interaction between beneficial fungi like Trichoderma species and plant hosts represents one of nature's most sophisticated defense partnerships. While Fusarium pathogens threaten crops worldwide, causing devastating root rot and wilt diseases, Trichoderma fungi have evolved as powerful biocontrol agents that not only directly antagonize pathogens but also "prime" plant immune systems for enhanced resistance. This multi-layered defense strategy transforms plants into fortified organisms capable of mounting rapid, robust responses against Fusarium attacks.
The Tripartite Molecular Recognition System of Trichoderma
Pattern Recognition and Initial Contact
When Trichoderma colonizes plant roots, it initiates a complex molecular dialogue through multiple recognition mechanisms. The fungus releases microbe-associated molecular patterns (MAMPs) including chitin oligosaccharides, cell wall fragments, and specialized proteins that plant pattern recognition receptors (PRRs) detect. However, unlike pathogenic interactions, this recognition leads to a carefully modulated immune response that enhances rather than damages plant health.
Elicitor Molecules: The Chemical Messengers
Trichoderma produces numerous elicitor compounds that trigger plant defense responses. Key elicitors include:
Hydrophobins - Small secreted proteins that activate ROS production and pathogenesis-related (PR) protein synthesis
Cell wall-degrading enzyme fragments - Oligosaccharides released during fungal metabolism that prime defense pathways
SM1 protein - A small extracellular protein from T. virens that specifically activates jasmonic acid pathway
Peptaibols - Antimicrobial peptides that trigger both local and systemic resistance responses
Dual Pathway Activation: ISR and SAR Working in Concert
Induced Systemic Resistance (ISR): The JA/ET Pathway
Trichoderma primarily activates induced systemic resistance through jasmonic acid (JA) and ethylene (ET) signaling pathways.
This process involves:
Initial Recognition: Root colonization by Trichoderma triggers JA biosynthesis in root tissues
Signal Amplification: JA activates transcription factors like MYC2 that regulate defense gene expression
Systemic Transmission: Mobile signals travel through the plant's vascular system to prime distant tissues
Defense Priming: Distal tissues become "primed" to mount faster, stronger responses upon pathogen attack
Research demonstrates that JA-deficient mutants lose Trichoderma-induced protection, confirming the essential role of this pathway. The PDF1.2 gene serves as a key marker for ISR activation, showing enhanced expression in Trichoderma-colonized plants.
Systemic Acquired Resistance (SAR): The SA Pathway
Simultaneously, Trichoderma can activate systemic acquired resistance through salicylic acid (SA) signaling.
This pathway:
Early SA Accumulation: Trichoderma interaction initially elevates SA levels in root tissues
NPR1 Activation: SA binding to NPR1 (Non-expressor of PR genes 1) allows this master regulator to enter the nucleus
PR Gene Expression: NPR1 activates pathogenesis-related genes including PR1, PR2, and PR5
Systemic Protection: SA-dependent signals spread throughout the plant, establishing broad-spectrum resistance
The temporal dynamics of pathway activation are crucial - studies show Trichoderma initially primes SA-regulated defenses to limit early pathogen invasion, then shifts to enhance JA-regulated responses that prevent pathogen establishment and reproduction.
MAPK Signaling: The Information Highway
Trichoderma MAPK Requirements
The fungus itself requires functional mitogen-activated protein kinase (MAPK) signaling to induce plant resistance. Research using tmkA gene knockout mutants in T. virens revealed that while these mutants colonize roots normally, they fail to trigger full systemic resistance. This indicates that Trichoderma must actively process and respond to plant signals through its own MAPK cascades to successfully prime plant defenses.
Plant MAPK Activation
In plants, Trichoderma-plant interaction activates multiple MAPK cascades:
MPK3/MPK6 pathway: Critical for defense gene expression and ROS production
MPK4 pathway: Involved in negative regulation to prevent excessive defense responses
Stress-responsive pathways: Including osmotic stress and wound response cascades
Reactive Oxygen Species
Controlled ROS Production
Trichoderma colonization triggers carefully regulated reactive oxygen species (ROS) production, including hydrogen peroxide (H₂O₂) and superoxide radicals. This oxidative burst serves multiple functions:
Antimicrobial Activity: ROS directly damage pathogen cell walls and membranes
Signal Transduction: ROS act as signaling molecules that activate downstream defense pathways
Cell Wall Reinforcement: ROS-mediated cross-linking strengthens plant cell walls against pathogen invasion
Antioxidant Balance
Critically, Trichoderma enhances plant antioxidant systems to prevent ROS-mediated self-damage. The fungus upregulates key antioxidant enzymes:
Catalase (CAT): Decomposes H₂O₂ to water and oxygen
Superoxide dismutase (SOD): Converts superoxide radicals to H₂O₂
Ascorbate peroxidase (APX): Uses ascorbic acid to neutralize H₂O₂
Glutathione peroxidase (GPX): Reduces organic peroxides using glutathione
This balanced approach allows beneficial oxidative signaling while preventing cellular damage that pathogens might exploit.
Metabolic Reprogramming for Defense
The Pentose Phosphate Pathway Enhancement
Trichoderma significantly enhances the plant's oxidative pentose phosphate pathway (OPPP), which provides:
NADPH production: Essential for antioxidant enzyme function and defense metabolite synthesis
Ribose-5-phosphate: Building blocks for nucleotides and aromatic amino acids
Erythrose-4-phosphate: Precursor for phenolic compounds and lignin
Ascorbate-Glutathione Cycle Optimization
The fungus optimizes the ascorbate-glutathione cycle by enhancing key enzymes:
γ-glutamylcysteine synthetase (γ-GCS): Rate-limiting enzyme for glutathione biosynthesis
L-galactono-1,4-lactone dehydrogenase (GalLDH): Final step in ascorbic acid synthesis
Glutathione reductase (GR): Regenerates reduced glutathione for continued antioxidant activity
Transcriptional Networks: Orchestrating the Defense Symphony
WRKY Transcription Factors
Trichoderma colonization extensively activates WRKY transcription factors, master regulators of plant immune responses.
Key WRKY proteins include:
WRKY33: Activated by chitin oligosaccharides and ROS, regulates antimicrobial compound production
WRKY70: Integrates SA and JA signaling pathways
WRKY22/29: Downstream targets of MAPK cascades that regulate pathogen response genes
Defense Gene Networks
Transcriptomic analyses reveal that Trichoderma treatment activates extensive gene networks involved in:
Cell wall modification: Genes encoding cellulases, xyloglucan endotransglycosylases, and lignin biosynthetic enzymes
Secondary metabolism: Pathways producing antimicrobial compounds, phytoalexins, and phenolic acids
Protein degradation: Proteases and peptidases that can degrade pathogen effectors
Transport processes: ABC transporters that export toxic compounds and import nutrients
Hormonal Crosstalk: Fine-Tuning the Response
SA-JA Antagonism and Synergy
The relationship between SA and JA pathways in Trichoderma-induced resistance is complex and context-dependent. While these pathways classically antagonize each other:
Early stages: SA and JA work synergistically to establish initial protection
Pathogen challenge: JA-mediated responses dominate against necrotrophs like Fusarium
Recovery phase: SA pathways help resolve inflammation and restore homeostasis
Ethylene's Modulatory Role
Ethylene serves as a crucial modulator, often working with JA to enhance resistance while also influencing the timing and magnitude of defense responses. The JA/ET signaling module is particularly important for resistance against necrotrophic pathogens.
Priming vs. Direct Activation: The Strategic Advantage
Defense Priming Concept
Rather than constitutively activating expensive defense responses, Trichoderma "primes" plant immune systems. Priming involves:
Chromatin remodeling: Making defense genes more accessible for rapid transcription
Protein pre-positioning: Accumulating defense-related proteins in inactive forms
Metabolic preparation: Pre-loading biosynthetic pathways with precursors
Signaling sensitization: Increasing sensitivity to pathogen-associated signals
This strategy provides fitness advantages by maintaining normal growth while enabling rapid defense deployment when needed.
Molecular Memory
Trichoderma treatment can establish transgenerational priming effects, where treated plants pass enhanced disease resistance to their offspring through epigenetic mechanisms. This molecular memory involves DNA methylation changes and histone modifications that maintain defense-related genes in primed states. (source)
Specificity Against Fusarium Pathogens
Targeting Fusarium Vulnerabilities
Trichoderma-induced defenses are particularly effective against Fusarium because they target specific vulnerabilities of these pathogens:
Cell wall degradation: Enhanced plant chitinases and β-1,3-glucanases directly attack Fusarium cell walls
Toxin neutralization: Upregulated detoxification enzymes can break down Fusarium mycotoxins
Root colonization interference: Physical competition and antibiosis prevent Fusarium root establishment
Vascular defense: Enhanced lignification and tylosis formation block Fusarium vascular invasion
Anti-Fusarium Metabolites
Trichoderma treatment stimulates production of specific anti-Fusarium compounds:
Phytoalexins: Species-specific antimicrobial compounds like camalexin in Arabidopsis
Phenolic acids: Including caffeic acid, ferulic acid, and chlorogenic acid that inhibit Fusarium growth
Flavonoids: Such as quercetin and kaempferol derivatives with antifungal properties
Clinical Applications and Future Directions
Agricultural Implementation
Understanding these molecular mechanisms enables more effective Trichoderma applications:
Timing optimization: Applying Trichoderma during critical plant developmental stages
Strain selection: Choosing Trichoderma strains with optimal elicitor profiles
Environmental considerations: Matching application conditions to maximize MAPK signaling
Integration strategies: Combining with other biocontrol agents for additive effects
Biotechnological Enhancements
Future developments may include:
Engineered elicitors: Synthetic versions of key Trichoderma signaling molecules
Transgenic approaches: Plants engineered with enhanced Trichoderma recognition capacity
Microbiome management: Optimizing soil microbial communities to support Trichoderma establishment
The Trichoderma-plant partnership represents a pinnacle of co-evolutionary adaptation, where beneficial microbes have developed ways to communicate with and enhance plant immune systems through sophisticated molecular mechanisms.
By simultaneously activating ISR and SAR pathways, modulating ROS production, reprogramming plant metabolism, and orchestrating complex transcriptional networks, Trichoderma transforms plants into resilient defenders against Fusarium and other pathogens.
This natural biocontrol system offers sustainable alternatives to chemical fungicides while providing insights into fundamental plant-microbe interactions.
As our understanding of these molecular mechanisms deepens, we can develop more effective, environmentally friendly strategies for crop protection that harness the power of beneficial microbes like Trichoderma.
The future of plant disease management lies not in overwhelming pathogens with synthetic chemicals, but in empowering plants with their own sophisticated immune systems through strategic microbial partnerships.
Sources:
https://apsjournals.apsnet.org/doi/10.1094/PDIS-08-19-1676-RE
https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2023.1145715/full
https://www.jstage.jst.go.jp/article/jsme2/39/4/39_ME24038/_article
https://apsjournals.apsnet.org/doi/10.1094/MPMI-07-14-0194-R
https://apsjournals.apsnet.org/doi/10.1094/PHYTO-09-18-0342-R
https://academic.oup.com/hr/article/doi/10.1093/hr/uhaf082/8069470
https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2013.00206/full



Comments